U.S. Drastically Revises Childhood Vaccine Schedule Under Trump Administration
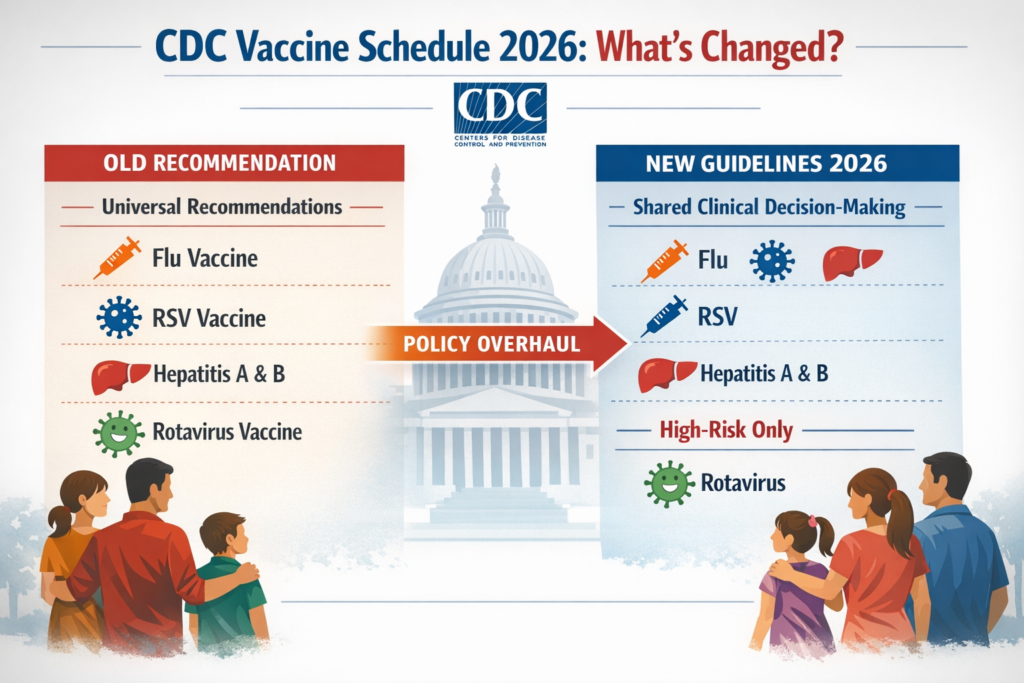
WASHINGTON, D.C. — In a major shake-up of public health policy, the Centers for Disease Control and Prevention (CDC) has dramatically revised the United States’ childhood immunization schedule to recommend fewer routine vaccines for all children, a move championed by President Donald Trump and supported by Health Secretary Robert F. Kennedy Jr.
Under the updated guidelines, the CDC will still advise universal vaccination against serious diseases such as measles, mumps, rubella, polio, pertussis (whooping cough), tetanus, diphtheria, Haemophilus influenzae type B (Hib), pneumococcal disease, varicella (chickenpox) and HPV — though even the HPV recommendation has been reduced from multiple doses to one shot in many cases. Meanwhile, vaccines that were previously recommended for all children — including shots for influenza (flu), COVID-19, rotavirus, RSV (respiratory syncytial virus), hepatitis A and B and certain forms of meningococcal disease — are now only broadly recommended for certain high-risk groups or left to shared clinical decision-making between parents and doctors.
The policy change stems from a Presidential Memorandum issued by Trump in December 2025, directing the Department of Health and Human Services (HHS) to examine childhood vaccine schedules in other developed nations and adjust U.S. recommendations accordingly. The CDC acting director signed a decision memorandum accepting these updated recommendations, which align U.S. practice more closely with vaccine schedules in countries like Denmark and others with fewer universal shots. Officials say the overhaul aims to increase public trust and allow more parental choice in vaccination planning, while maintaining insurance coverage for all listed vaccines.
Public health experts and medical associations have reacted strongly against the new schedule, warning that removing broad recommendations for key vaccines — especially the annual flu shot — could lead to lower vaccination rates and increase the risk of preventable disease outbreaks, particularly as the U.S. navigates a severe flu season. Critics also argue that the changes were implemented without the usual transparency and expert advisory review, raising concerns over scientific rigor in central public health policy decisions.
Supporters of the revision argue that many peer nations recommend fewer routine vaccines yet maintain strong health outcomes, and that the new framework empowers families and clinicians to make decisions tailored to individual needs. However, medical professionals emphasize that broad, science-based recommendations have historically helped eradicate or control serious diseases and urge caution as the nation adjusts to the new guidance.





